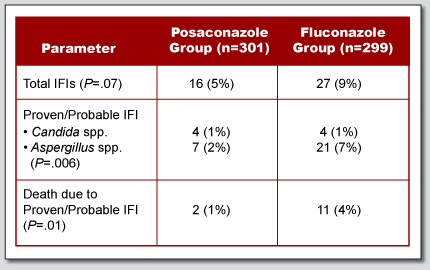
A randomized, double-blind trial compared posaconazole and fluconazole prophylaxis in patients with GVHD.
Treatment regimen:
fluconazole 400 mg qd or posaconazole 200 mg tid
-
Assessment:
at end of treatment + 7 days and at 16 weeks
-
Mean days on therapy:
fluconazole, 77 d; posaconazole, 80 d
|
|
- Limitations:
- Posaconazole exposure is greatly improved when taken with a fatty meal or nutritional supplement, which may have led to selection bias. (Serum concentrations were not routinely measured.)
- Many patients (posaconazole group, 7%; fluconazole group, 10%) had a positive baseline galactomannan result suggesting that they were undergoing pre-emptive therapy rather than true prophylaxis.
- Overall mortality was not reduced.
- Clinical failure rates were similar in both groups.
Ullmann AJ, Lipton JH, Vesole DH, et al. N Engl J Med. 2007;356(4):335-347.
|
| |
|
 |



